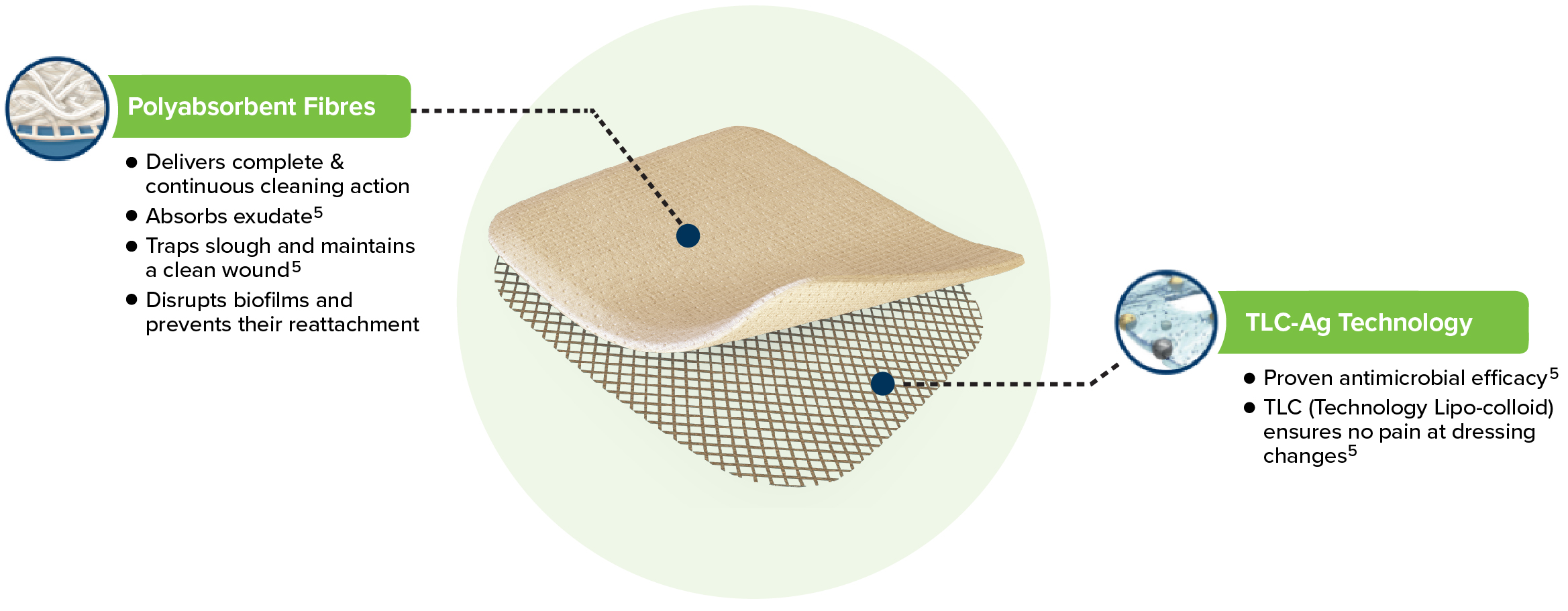
UrgoClean Ag is a soft-adherent dressing pad with polyabsorbent fibres and TLC-Ag healing matrix.
- Continuously cleans the wound: Polyabsorbent fibres trap and retain exudate, slough and debris.1
- Proven antimicrobial efficacy: Silver ions provide broad spectrum antibacterial activity, including MRSA2
- Antibiofilm action: combined cleaning and antimicrobial action defeats biofilm and prevents their reattachment2
- TLC-Ag ensures atraumatic removal and pain free dressing changes1
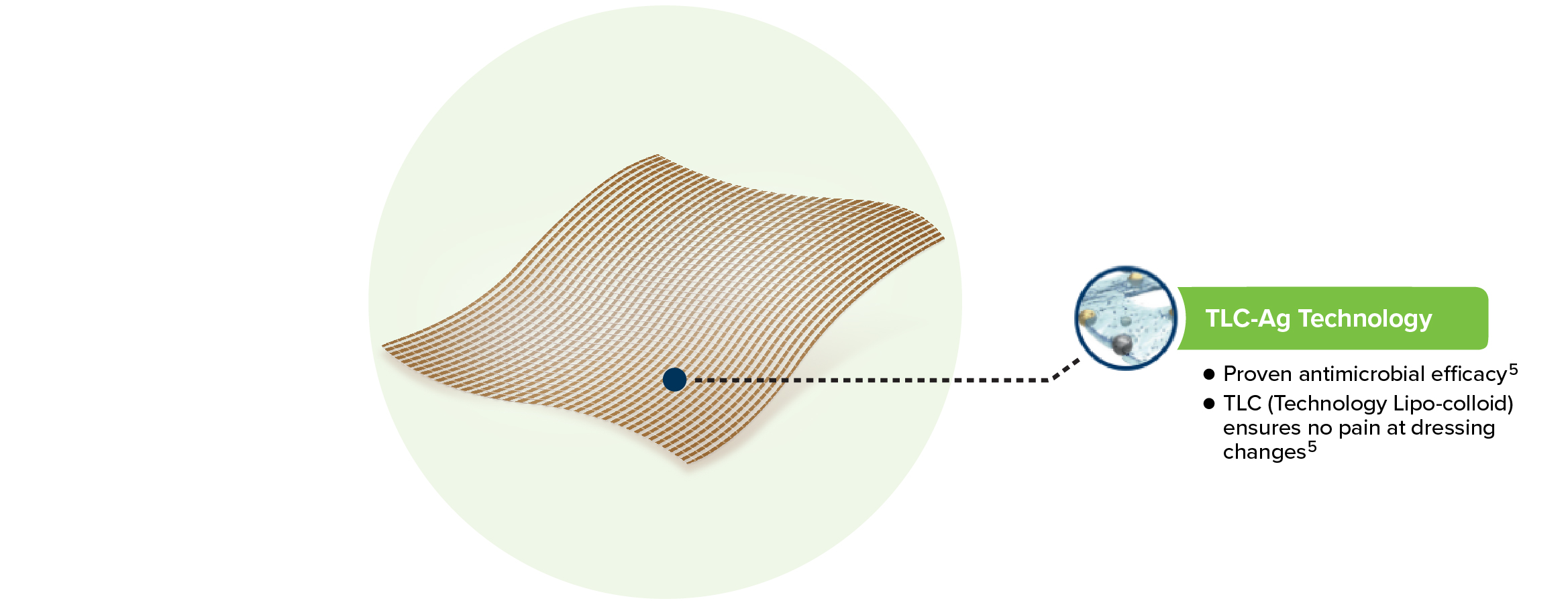
UrgoTul Silver is a non-adhesive, non-occlusive and highly conformable contact layer dressing with TLC-Ag healing matrix.
- Proven antimicrobial efficacy: Silver ions provide broad spectrum antibacterial activity, including MRSA.2
- TLC-Ag ensures atraumatic removal and pain free dressing changes.1
UrgoClean Ag is indicated for the local treatment of chronic (leg ulcers, pressure ulcers, diabetic foot ulcers) and acute (burns, traumatic wounds, surgical wounds), exudative wounds at risk or with signs of local infection, from the debridement stage.
Contraindications:
- Known sensitivity to silver.
- UrgoClean Ag is not suitable as a surgical sponge for heavily bleeding wounds.
- Do not use UrgoClean Ag in combination with hydrogen peroxide, organomercuic antiseptics or hexamidine.
- Do not use on patients undergoing Magnetic Resonance Imaging (MRI) examination.
Indications:
- Non to low exuding wounds, infected or at risk of infection
- Can be combined with an absorbent layer
- Can be used in cavity wounds
UrgoTul Silver is indicated for the local treatment of wounds at risk or with signs of local infection: chronic wounds (pressure ulcers and leg ulcers) and acute wounds (partial thickness burns, dermabrasions, traumatic wounds, surgical wounds, etc…).
Due to the non-adhesive mature of the dressing, UrgoTul Silver is recommended in the treatment of wounds in which the skin around the lesion is weakened.
Contraindications:
- Known sensitivity to silver and other ingredients of the dressing
- Do not use on patients undergoing Magnetic Resonance Imaging (MRI) examination.
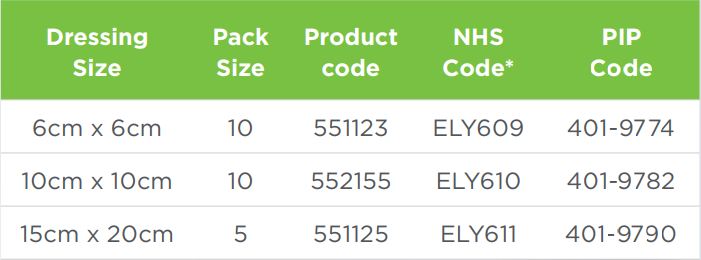
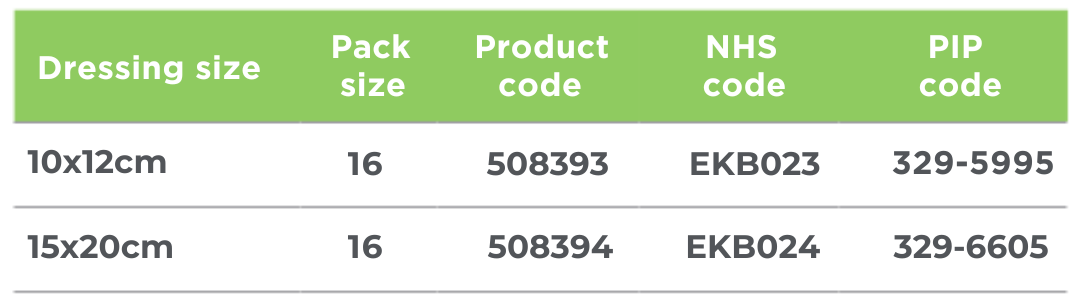
- Wound preparation:
- Clean the wound with normal saline. In the event of prior use of an antiseptic (except contraindicated antiseptics), rinse the wound carefully with normal saline before applying the dressing.
- The use of UrgoClean Ag does not dispense with the need for associated mechanical debridement when required.
- During desloughing, the wound may appear to get larger due to gradual elimination of slough.
- Dressing application:
- Remove the protective tabs.
- Apply with the micro-adherent side of UrgoClean Ag in contact with the wound and its edges.
UrgoClean Ag can be cut using sterile scissors to adjust the dressing size to fit the wound if necessary.
- If necessary, cover UrgoClean Ag with a secondary dressing suitable for the wound location and level of exudate.
- Secure in place with a suitable bandage or tape.
- Apply a compression bandage where prescribed.
- Dressing changes:
UrgoClean Ag should be changed every 1 to 2 days during the wound desloughing phase, then as often as required (up to 7 days) depending on the exudate volume and the clinical condition of the wound. The maximum treatment duration with UrgoClean Ag is 1 month.
Precautions for use:
- Treatment with UrgoClean Ag should be performed under medical supervision.
-
The use of this dressing does not negate the need for appropriate systemic anti-bacterial treatment of infected wounds in line with local policy.
- The silver-impregnated micro-adherent healing matrix (TLC-Ag) in UrgoClean Ag sticks to latex surgical gloves. It is therefore recommended that the dressing be handled carefully, avoiding any contact with the micro-adherent side, or using sterile tongs.
- The concomitant use of other local treatments is not recommended.
- Avoid contact with electrodes or conductive gels during electronic measurements, such as EEG or ECG recordings.
-
Clinicians/Healthcare professionals should be aware that there is very limited data on prolonged and repeated use of silver containing dressings, particularly in children and neonates.
-
In the absence of any specific clinical data, use in pregnant or breast-feeding women, is not recommended.
- UrgoClean Ag must not be used during hyperbaric oxygen chamber therapy without an oxygen mask (risk of combustion due to the presence of fat). This contraindication does not apply for hyperbaric oxygen chamber therapy with an oxygen mask if the oxygen concentration inside the chamber is less than 25% and if UrgoClean Ag is not applied on the area over which the mask is placed.
- Sterile individual packaging, for single use. Reuse of a single-use dressing can lead to risks of infection.
-
Check that the sterile protector is intact before use.
- Do not re-sterilise.
- For disposal, refer to the existing protocol. Discard any unused parts of the dressing.
- Cleanse the wound with saline solution. If an antiseptic is first used, rinse the wound thoroughly with saline solution before applying UrgoTul Silver.
- If needed, UrgoTul Silver can be cut using sterile scissors to match the size of the dressing to the wound.
- Remove the protective wings from the dressing.
- Apply the UrgoTul Silver dressing directly onto the wound.
- Cover UrgoTul Silver with a secondary dressing (sterile pad suitable for the absorption of exudate, to be secured in place with a fixing bandage).
- Apply a compression bandage when prescribed.
- The UrgoTul Silver dressing can be changed every 1 to 3 days depending on the wound treated and the course of its healing.
- The maximal duration of the treatment with UrgoTul Silver is one month.
Precautions for use:
- Treatment with UrgoTul Silver should be carried out under medical supervision.
- Use of this dressing does not negate the need for appropriate systemic antibacterial treatment for infected wounds, in line with local policy.
- UrgoTul Silver can adhere to surgical gloves (latex). Consequently, it is recommended that the gloves be moistened with normal saline in order to make it easier to hold the dressing.
- Concomitant use with other local treatments is not recommended.
- Do not use in pregnant or breastfeeding women, newborn or premature babies in the absence of any specific clinical data.
- Avoid contact with electrodes or conductive gels during electronic measurements, e.g. EEG or ECG.
- Do not use in a hyperbaric chamber.
- Single-use, individual and sterile dressing: The reuse of a single use product may entail risks of infection.
- Check that the sterility protector is intact before use. Do not use if the packaging is damaged.
- Do not re-sterilise.
Get in touch with your local representative for more information about the UrgoClean Ag or UrgoTul Silver Range
References:
1. Dalac S., Sigal L., Addala A., et al Clinical evaluation of a dressing with poly-absorbent fibres and a silver matrix for managing chronic wounds at risk of infection: a non comparative trial. J Wound Care, Vol 25, No 9, September.
2. Desroche N., Dropet C., Janod P., Guzzo J., Antibacterial properties and reduction of MRSA biofilm with a dressing combining polyabsorbent fibres and a silver matrix. J Wound Care, Vol 25, No 10, October 2016.
3. Lazareth T, Meaume S, Sigal-Grinberg M-L, et al. The role of a Silver Releasing Lipido[1]colloid Contact Layer in Venous Leg Ulcers Presenting Inflammatory Signs Suggesting Heavy Bacterial Colonization: Results of a Randomized Controlled Study. WOUNDS 2008;20(6):158-166.
4. Meaume S, Dissemond J, et al, Evaluation of two fibrous wound dressings for the management of leg ulcers: Results of a European randomized controlled trial (EARTH RCT). J Wound Care , Vol 2, No 3; March 2014, 105-116.
5. Dissemond J, Dietlein M, NeβELER L, Funke L, Scheuermann O, Becker E, Thomassin L, Möller U, Bohbot S, Múnter KC. Use of a TLC-Ag dressing on 2270 patients with wounds at risk or with signs of local infection: an observational study. J Wound Care. 2020,Mar 2;29(3):162-173. doi: 10,12968/jowc2020.29.3.162. PMID: 32160091.
6. UrgoClean Ag data on file.
7. Desroche N, et al. Characterization of the antimicrobial spectrum and anti-biofilm activity of a new silver-containing dressing with poly-absorbent fibres and antimicrobial silver matrix. Poster EWMA May 2016.
8. Desroche N, et al. Evaluation of in vitro anti-biofilm activities of a new poly-absorbent dressing with a silver matrix and a silver-containing CMC dressing. Poster EWMA May 2017.
9. Desroche N, et al. Evaluation of in vitro anti-biofilm activities of two dressings with poly[1]absorbent dressing fibres and a DACC-coated dressing. Poster EWMA 2017.
10. Wounds UK (2021) Best Practice Statement: Use of silver dressings in wound care. Wounds UK, London.
11. Desroche N, et al. Comparison of in vitro anti-biofilm activities of a new poly-absorbent dressing with a silver matrix and a silver-containing CMC dressing. Poster EWMA, May 2017.

